by Ryan Durán
The future of pharmaceuticals lies in automation and digitalization. With the introduction of Pharma 4.0 by the International Society of Pharmaceutical Engineers (ISPE), the roadmap for innovation and the digitalization of production processes is on its way.
Currently, many pharmaceutical companies automate the monitoring and control of production processes, particularly in the manufacturing of active pharmaceutical ingredients (API) and their conversion into consumer products. However, facilities still rely on manual data recording, indicating a substantial opportunity for improvement. A full-scale digital transformation in one of America’s largest industries is poised to have a profound impact, a shift that ISPE is actively promoting.
Implementing additional automation technologies poses unique challenges for the pharmaceutical sector. The pharmaceutical industry must adhere to documentation practices overseen by the Food & Drug Administration (FDA) as well as other international organizations like the European Medical Association (EMA). According to ALCOA+ data integrity criteria from the FDA, documentation must be attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available. The FDA also requires pharmaceutical facilities to comply with Good Manufacturing Practice (GMP) and Good Documentation Practice (GDP) to ensure that products remain safe, pure, and effective as they are handled by manufacturers, processors, and packagers.
From an API’s inception to the consumer-facing labelling, there is a wide variety of manufacturing processes in pharmaceuticals, with each step regulated by the FDA. Any automation must be compliant with the FDA regulations such as GDP and GMP. This is a factor to be conscious of when finding an automation consultant or integrator: how accustomed are they to the rigorous documentation standards required by the FDA? Cutting corners in this area can lead to serious legal and regulatory consequences. Partnering with like-minded automation specialists like Enterprise Automation who understand these compliance demands can help eliminate that risk and ensure your systems are designed and validated to meet all requirements.
GDP is critical for all official documentation within FDA-regulated facilities. This includes batch records, internal and external testing documents, lab journals, and any content requiring verifiable data integrity. GDP also plays a vital role in system validation, which ensures that products and processes are thoroughly documented and remain usable over time. System validation is a structured process that confirms a computer system operates as intended, according to its design specifications, and does so consistently and reliably. Validation encompasses various parts of the control system and necessitates comprehensive documentation and testing.
ISPE and Good Automated Manufacturing Practice (GAMP) guidelines describe the automated system validation process in detail. A widely used approach within this framework is the V-model, which organizes system validation activities based on the system’s complexity. The V-model visually represents the relationship between specification development and verification testing. Specifically, the process begins with writing the specifications for an automated system (the left side of the V), followed by programming the system (the bottom of the V), and concludes with testing the system against its specifications (the right side of the V). Throughout this process, maintaining proper documentation is critical.
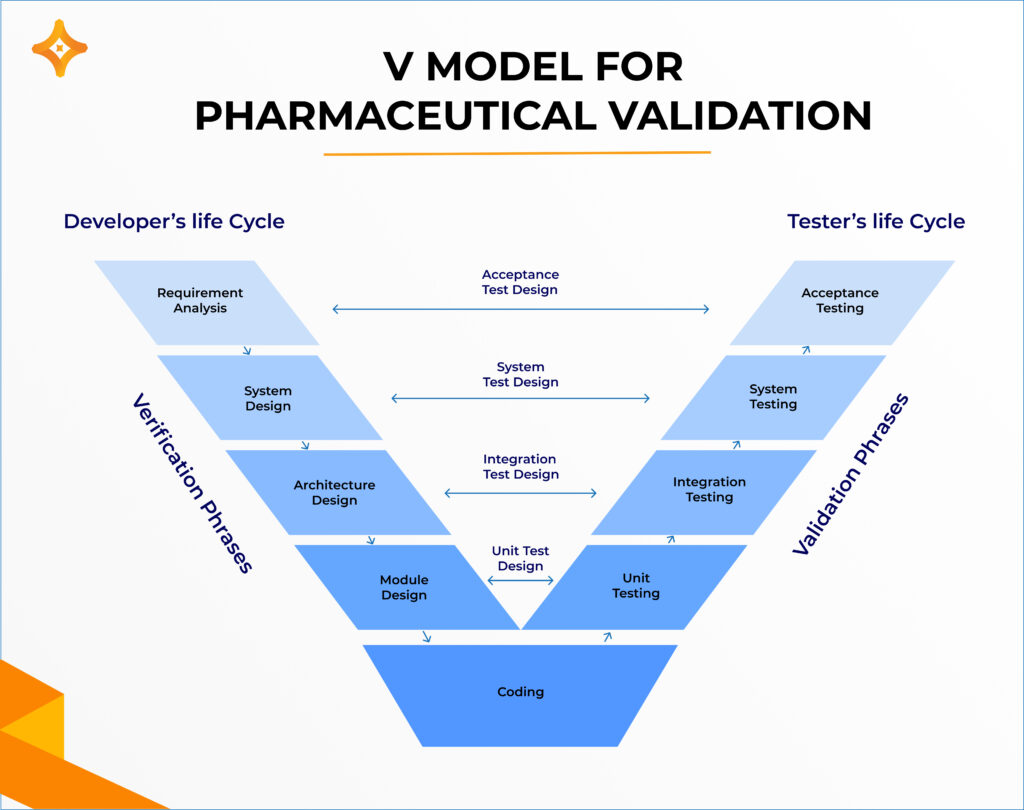
Once a system is validated, components such as code segments or SCADA graphics can be reused across different applications within the system. However, these validated systems must be accompanied by appropriate documentation, including user requirement specifications and model/version numbers. Tracking model and version numbers is essential, as validated systems can evolve over their lifecycle; thus, all changes must be meticulously documented. For reusable code modules or SCADA graphics, changes are typically monitored through model, version, or revision numbers, which are maintained in document control or quality management software to ensure compliance and traceability.
GMP standards necessitate the long-term storage of data, which can present significant challenges due to the volume of information generated. While some facilities maintain physical records that are digitized later, modern control systems can facilitate entirely digital record-keeping. For instance, if a facility records a process variable every 30 seconds, this results in over one million data points annually that would otherwise require manual entry. By digitizing and automating data storage, facilities can save thousands of hours while enhancing security through automated backups and minimizing the risk of error associated with manual data entry. A robust SCADA system enables efficient data collection and reporting from equipment and instruments.
The applications of automation vary widely, from small-scale manufacturing to product delivery, and depend on the specific pharmaceutical products involved. In small-scale operations, production quantities can range from micrograms to grams, particularly during research and development testing. Small-scale production can be relatively simple, and manual controls may be adequate. However, when batch production becomes more frequent, efficiency becomes more important, making automation a more valuable operational advantage. Collecting data manually from these systems can be labor-intensive and prone to error.
For instance, consider a small-scale spray-dried dispersion (SDD) reactor. Although it can be operated manually, integrating a small-scale automated system could significantly enhance control and data collection. An automated system would provide a display to monitor critical parameters such as flow rate, pressure, temperature, and duration. Additionally, it would include system controls and alerts for deviations from desired operating setpoints. This automation reduces the risk of producing low-quality SDDs due to operator inattention or equipment failure, ultimately minimizing waste of active pharmaceutical ingredients (APIs) and excipients.
Mid-sized facilities, which manufacture at the gram to kilograms level, often require flexibility to accommodate a variety of clients. An example of this is custom API manufacturing for pre-clinical studies or clinical trials. Automation is commonly employed in these scenarios, as reactors must efficiently handle both high and low volumes of product. Mid-sized facilities can implement multiple processes or recipes that utilize operator inputs to tailor production quantities. Utilizing properly validated code modules and SCADA graphics allows for easier reconfiguration of existing hardware to accommodate new client requirements, reducing the costs and complexities associated with validation each time the system is reorganized. Given that validation can be both time-consuming and expensive, it is vital to execute it effectively at each stage.
At the commercial scale, automation becomes indispensable for maintaining process consistency and ensuring proper data management. Commercial operations often involve dozens to hundreds of inputs and outputs, and automation enables continuous operation with minimal operator intervention. Reducing the reliance on manual operation is critical, as delays in operator action can lead to increased system downtime. Furthermore, a well-configured and tested SCADA historian ensures that all process data is accurately recorded and preserved.
As automation becomes increasingly integral to the pharmaceutical sector, partnering with a skilled systems integrator is essential for developing automation systems that optimize resources over time. Without the expertise of a qualified systems integrator, facilities risk accumulating technical debt, poorly documented programs, and systems that fail to meet operational needs. A competent systems integrator can facilitate seamless workflows, minimize human error, and support facility growth.
Enterprise Automation specializes in designing and validating control systems while adhering to regulatory standards within the life sciences sector. Regardless of your company’s size and technological capabilities, we work within your budget and personnel constraints to deliver solutions that provide lasting value. Reach out to us to secure a robust SCADA system and the ongoing support needed to maintain it for years to come.